2. 上海交通大学 生命科学技术学院 上海 200240
2. School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai 200240, China
萜类化合物是由异戊二烯单元组成的有机化合物,已知结构超80 000种[1],具有多样的生物活性。根据异戊二烯单元数量的不同,萜类化合物被分类为单萜、倍半萜、二萜、二倍半萜、三萜、四萜等,具有复杂多变的空间结构和多样的理化性质与生物学功能。例如,倍半萜青蒿素是具有抗疟活性的诺(贝尔)奖级药物,二萜紫杉醇是抗癌领域的“明星”药物,三萜角鲨烯具有保湿和渗透功效,四萜虾青素具有显著抗氧化作用。这些独特的性质使得萜类化合物在医药、香料、化妆品、食品添加剂等领域有广泛应用。
随着合成生物学的蓬勃发展,萜类化合物的研究与开发正逐步成为推动生物科技创新、加速产业升级的重要力量。然而,在这一进程中,也存在着诸多挑战。一方面,随着基因组测序技术的日益成熟,互联网上汇聚了海量的未鉴定合成酶资源,如何高效挖掘并充分利用这些宝贵资源成为亟待解决的问题。另一方面,尽管萜类化合物的高产菌株的构建已不再遥不可及,但如何实现高产菌株的规模化生产,推动其产业化制造与商业化应用,仍是一项必须面对的关键挑战。
1 萜类化合物的高效发现在“第三次生命科学革命”的浪潮中,天然产物作为药物的重要来源,其发掘工作正经历着深刻变革。面对全球性多药耐药问题的挑战和现代药物开发的需求,传统的依赖生物样本分离提取的方法已难以满足当前需求[2]。随着计算能力的提升、基因组层面技术的发展、微生物培养技术的成熟,以及高通量自动化平台的建立,天然产物在药物先导化合物的探索中再次受到重视,并开辟了新的机遇[3]。
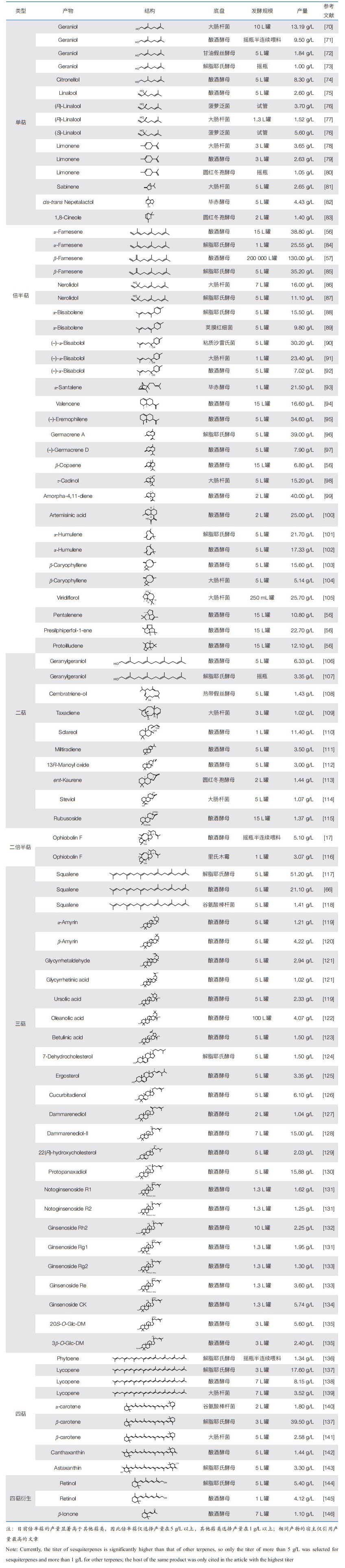
萜类化合物的高效发现,作为生物学科的关键研究方向之一,不仅能够丰富天然产物资源,更能促进相关产业的技术革新和产品升级。目前,主要通过3种方法来挖掘萜类化合物:直接从天然来源提取、激活沉默基因(簇)和异源表达[4]。这些方法的综合应用极大地促进了萜类化合物的发现。然而,已鉴定萜类合酶和萜类化合物仍然只是自然界中庞大萜类资源的一小部分。当前研究的焦点是如何突破萜类化合物挖掘过程中的“三低”难题——低产量、低结构新颖性和低研究效率[5],并在此基础上发展出更高效的策略来进行挖掘(图 1)。
|
| 图 1 突破萜类化合物挖掘难题的策略 Figure 1 Strategies to break through challenges in terpene mining processes |
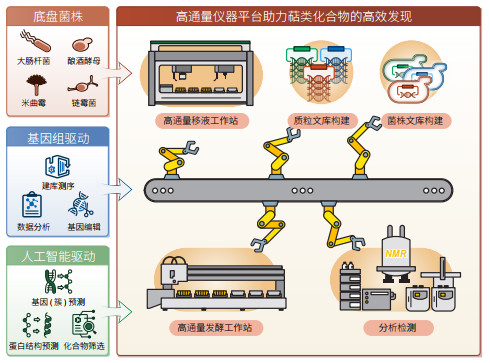
异源表达是一种通过引入外源生物合成基因(簇)到具有成熟遗传操作的生物体中,以揭示其功能的方法。这种方法解决了原始宿主不可获得和遗传操作困难的问题,被广泛应用于萜类化合物的挖掘和生物合成途径的解析,并实现了多种明星萜类分子的从头合成(表 1)。选择异源宿主时需综合考虑其生长速度、代谢背景、遗传操作便利性和对目标化合物的耐受性[6]。常见的宿主包括大肠杆菌、酵母、链霉菌、丝状真菌和模式植物烟草等。然而,受限于异源宿主的合成能力,仅能鉴定丰度高的产物,而丰度低的产物易被忽略或难以获取,这限制了对萜类合酶及其后修饰酶的全面认知[7]。因此,改造异源宿主开发高效供给萜类前体的底盘菌株,提高产物的丰度和多样性,对新萜类化合物的挖掘具有重要意义。
|
(1)大肠杆菌底盘。大肠杆菌因其遗传操作简便和生长快速而备受青睐。利用“定向合成代谢”的概念,基于体外催化的结果指导体内代谢途径的改造,研究人员构建了高产异戊烯焦磷酸(IPP)和烯丙基焦磷酸(DMAPP)的大肠杆菌底盘[25]。在此基础上,通过组合不同异戊二烯前体和萜类合酶充分释放真菌来源萜类合酶FgGS和FgMS的合成潜力[26],研究人员检测到50种萜类化合物,鉴定了包括3种新骨架的8个新化合物。
(2)酵母底盘。酵母作为最易操作、研究透彻的真菌生物,已被广泛应用于萜类化合物的挖掘[27]。利用增强了内源甲羟戊酸(MVA)途径的酵母底盘菌株,研究人员高通量系统挖掘了74个真菌嵌合萜类合酶,其中34个具有活性,共产生了24种二萜和二倍半萜化合物,包括2种新的二倍半萜[28];并在此基础上,进一步颠覆性发现了非角鲨烯来源的三萜合成全新途径[29]。该策略还可以运用到植物的萜类合酶的挖掘中,通过在高效底盘中表征艾蒿来源的29个萜类合酶,研究人员发现了一个新倍半萜骨架和具有优秀驱虫活性的艾蒿醇(Intermedeol)[30]。
(3)丝状真菌底盘。丝状真菌因其内含子正确识别剪切和蛋白分泌能力,是真菌源萜类生物合成基因(簇)异源表达的理想宿主。在基于微生物高产底盘的天然产物创新挖掘策略[31]的基础上,研究人员进一步提出“一把钥匙开一把锁”的研究理念,选用酿造行业中最古老、应用最广泛的米曲霉[32]作为近源宿主挖掘了5株真菌的全部萜类合成基因簇,并构建了高效通用底盘,解决了异源表达适配性低和产量低的问题[16]。
(4)链霉菌底盘。链霉菌能够合成种类繁多、数量庞大的次级代谢产物,不但具有合成天然产物所需要的各种前体,还具备多类型产物耐受性和完善的修饰系统。链霉菌不仅可以用于萜类合酶的挖掘,还可用于细胞色素P450等后修饰酶的表征[33]。通过删除高产链霉菌中的阿维菌素及萜类等内源代谢物相关基因,简化遗传代谢背景和避免前体竞争,研究人员在异源表达细菌来源沉默萜类合酶时,成功鉴定到13个新萜类化合物[34-36]。
(5)植物底盘。植物是天然药物的重要来源,具备精细分隔的细胞结构以支持膜定位外源酶的功能,同时拥有全面的翻译后修饰系统,并具有安全性高、培养成本低等特点[37, 38]。利用模式植物烟草,研究人员以底物喂养和建立筛选底盘2种方式鉴定了紫杉醇合成途径的关键化合物和基因,实现了关键中间体baccatin Ⅲ在烟草中的从头合成[14],展示了植物宿主挖掘植物来源复杂萜类生物合成基因(簇)方面的优势。
1.2 基因组与高通量协同优化挖掘策略随着测序技术的飞速发展,全球基因组数据量激增,海量的基因组数据沉淀在互联网,亟待研究人员的挖掘,这使得基因组深度挖掘逐渐成为探索生命奥秘的一把钥匙。它能够直接从基因组数据中挖掘潜在的生物合成基因簇(BGC),以其独特的视角透视生物体内在的遗传信息,揭示微生物和植物中未被充分利用的化学多样性,扩展天然产物高效发现的深度。
然而,天然产物的高效发现并非易事,它依赖于速度与效率的双重提升。生物信息学的分析虽然提供了海量的信息,但其中不乏冗余,需要大量的实验验证来去伪存真。目前,数十万个分析归档的BGC中,仅不到0.25%进行过实验表征和产物确定[39],这一现状无疑制约了天然产物发现的步伐。
为了打破这一瓶颈,自动化高通量技术应运而生。它通过海量的工程化试错实验,取代传统的劳动密集型研究范式,能够快速、低成本、多循环地完成“设计—构建—测试—学习”的闭环,成为天然产物的自动化高效挖掘和高效构建高附加值化合物细胞工厂的解决方案[4]。目前,国内外多家企业及科研院校均已建成高通量筛选平台[40-42],实现了自动化基因编辑、菌株构建和高通量菌株筛选等功能,提高了天然产物挖掘的效率。
利用基于米曲霉底盘的高通量平台,研究人员系统性挖掘了5株丝状真菌中的萜类BGC,实现了从质粒构建、基因簇重构到活性筛选的全流程自动化实验,将39个萜类BGC自下而上重构至208个突变株中,检测到185个萜类产物,将实验周期缩短至29天,在活性筛选阶段发现了具有显著抗炎活性的二倍半萜Mangicol J并揭示了其生物合成机制[16]。在灵芝酸研究中,研究团队利用酿酒酵母高通量基因编辑平台,一次性构建了158个可能的细胞色素P450过表达质粒库,经3轮迭代菌株构建,挖掘到2种能够将Ⅰ型灵芝酸转化为Ⅱ型灵芝酸的P450,成功解析了灵芝酸生物合成途径中关键酶[20]。该创新性萜类挖掘策略结合基因组挖掘、高效底盘和高通量平台,不仅突破了传统挖掘方法通量低的限制,更为天然产物的发现与利用开辟了新的道路。
1.3 人工智能革新挖掘方式现代科学研究中,人工智能(AI)以其卓越的数据处理能力和模式识别技术,为天然产物的发现与研究带来革命性的突破。机器学习与深度学习的快速发展,不仅使研究人员能够高效地处理和解析海量的化学、生物学和药理学数据,还能够精确预测化合物的生物活性及潜在毒性。这一技术革新覆盖了从基因组和代谢组挖掘、结构表征到靶点和生物活性预测等多个关键环节[43, 44],引领着天然产物研究向更深层次的科学探索迈进。
在基因组挖掘领域,预测BGC是发现复杂次级代谢产物微生物的关键步骤。传统的BGC预测算法,如CLUSEAN、SMURF和antiSMASH等,能够在一定程度上识别与已知生物合成途径高度相似的区域[43],但在面对未知的全新基因簇时力不从心。相比之下,机器学习算法在此类任务中有显著优势。例如,Moore等[45]整合了自动化机器学习框架AutoGluon-Tabular和来自拟南芥的多组学数据,以预测参与植物次生代谢物如萜类、生物碱和酚类的BGC,鉴定了拟南芥中1 220个此前功能未知的基因。又如,Liu等[46]利用antiSMASH和DeepBGC同时分析马铃薯致病菌疮痂链霉菌的基因组,后者预测到额外的112个BGC,展示了人工智能在新型基因簇识别方面卓越的性能。
除了BGC预测外,蛋白质功能预测也是天然产物发现的重要一环。在这一领域,AlphaFold作为一款深度学习软件,成功模拟了氨基酸之间的复杂相互作用,并准确预测蛋白质的最终折叠形态[47]。AlphaFold到AlphaFold3[48]的迭代更新,不仅提升了预测的精度和效率,也为靶点治疗、新药研发,以及合成生物学等领域提供了重要的工具支持。值得一提的是,AlphaFold在蛋白质功能预测方面的应用,为萜类合酶的预测结构提供了高精度的快速解决方案。研究人员可以借此技术,指导进行蛋白质功能预测,从海量的蛋白数据库中迅速筛选出具有巨大潜力的候选蛋白,为后续的湿实验提供有力的指导,从而挖掘出全新的同类萜类合酶[29]。
新天然产物功效和靶点不明,且活性实验耗时冗长,阻碍了新药研发的进程,AI助力的天然产物高通量筛选可打破这一局面。一方面,AI通过整合和学习化合物结构与毒性、活性之间的关联,通过识别相似药效团预测新化合物理化性质和生物活性。另一方面,通过AI算法可以从多层面多角度大规模预测天然产物与潜在靶点间的相互作用[49-51],加速苗头化合物的发现,促进萜类化合物高效发现走向新药研发的进程。机器学习技术,如支持向量机(SVM)、随机森林(RF)、贝叶斯等算法已被成功应用于药物发现阶段的化合物筛选环节,帮助改善药物活性、预防药物副作用,并发现新靶点[52]。
尽管AI在药物研发领域,尤其是先导化合物的发现和优化上取得了大量进展,但在萜类化合物领域,目前成功应用案例尚少。在未来的研究中,AI有望从萜类化合物中挖掘出更多的新药候选物,为人类的健康事业贡献力量。
2 萜类化合物的产业化制造在萜类化合物的产业化制造领域,传统方法如化学合成、植物提取及天然含目标化合物的微生物发酵,虽各有其应用,却均面临显著局限。化学合成虽能实现大量生产,但步骤繁琐、能耗高且伴随有害副产品,对环境保护和可持续发展构成挑战。植物提取受限于生长周期、地域分布及含量有限,难以满足大规模生产需求。天然微生物发酵则因产量低、提取成本高而应用受限。
随着合成生物学技术的蓬勃发展,萜类化合物的产业化制造迎来了革命性发展。科学家通过巧妙设计并改造细胞工厂,成功将可持续的生物质能转化为具有复杂化学结构和强效活性的特定萜类化合物,不仅弥补了传统制造方法的短板,更开辟出一条绿色、可持续且高效的产业化新路径。
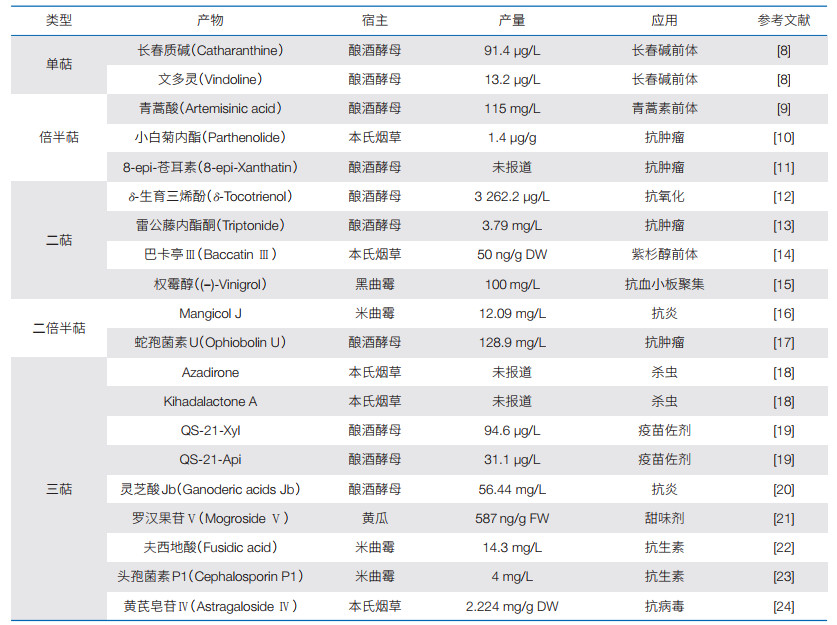
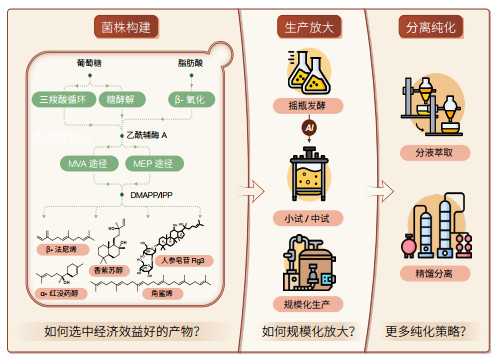
2.1 合成生物学助力萜类化合物生物制造在萜类化合物生物制造产业化进程中,萜类化合物细胞工厂的产品获取路径涵盖菌株构建、生产放大、分离纯化三大关键环节(图 2)。这一过程的本质在于,要确保细胞工厂具备超凡的生产速率,力求达到甚至超越植物提取、化学合成等传统工艺的水准。尽管两相发酵以进行原位萃取的策略可以显著提高小分子萜类的生产水平[53],但为了比较容易地纯化到最终产品,萜类化合物的生产滴度至少需达到克级每升的水平(表 2),这无疑对萜类化合物的产业化提出了严苛的要求。针对萜类化合物的生产速率这一核心瓶颈,科学家们开发出一系列创新策略。
|
| 图 2 萜类化合物细胞工厂的产品获取路径及存在的瓶颈 Figure 2 Product access pathways and bottlenecks in terpenoids cell factories |
(1)生物合成宿主的选择。随着CRISPR基因编辑技术的精进和全基因组序列信息的丰富,研究人员采用系统生物学方法对微生物制造工厂进行改良和创新,以实现萜类的高效合成。其中,大肠杆菌[54]、酿酒酵母[1]作为研究最为深入、代谢背景最为清晰的模式菌株,已实现多种萜类的高产。特别是酿酒酵母,凭借其表达高等真核生物基因的能力、单细胞组成的便捷操作性,以及实验室适应性进化赋予的可控性突变[55],成为萜类化合物生物制造的主力军。
(2)生物合成途径的重建。酿酒酵母中萜类化合物的合成以自身天然存在的MVA途径为核心,分别向上游的乙酰辅酶A供给和下游萜烯单体DMAPP/IPP利用进行延伸。通过增强整个MVA途径[56],全局代谢网络调控增加[57]或者多元化[58]乙酰辅酶A的供给,抑制旁路的竞争代谢途径使更多的萜烯单体流向最终产物[59, 60],平衡调控合成途径中的辅因子和能量供给[61]等策略,研究人员实现了诸多萜类化合物的高产。
(3)生物合成酶的工程改造。萜类合成途径下游的修饰酶是萜类结构复杂多样的关键。在原生生物体内,这些酶不仅催化特定反应,且整个途径受到复杂的机制调控,使其直接进行异源表达时,会造成生物合成步骤功能解耦,以及合成不良副产物等风险。通过酶工程实现理性设计[62],可以极为有效地提高酶的表达水平、催化效率和反应特异性。与此同时,可借鉴其他合成途径中的多酶级联自组装策略[63, 64],实现上下游途径的强耦合,以及解决底物传递和代谢流不稳定等问题。
(4)生物合成的靶向定位。萜类化合物结构的多样性极易引发细胞毒性,限制单萜的产量提升[65];并且,随着碳链延长造成的亲脂性的增强,三萜和四萜趋向于在特定亚细胞区室中积累。通过将整个生物合成途径定位到亚细胞器(线粒体[66]、过氧化物酶体[67]等),能够实现对中间毒性产物的区室化隔离,并对亚细胞器中丰富的乙酰辅酶A加以利用。此外,脂滴工程[68]储存毒性疏水性萜类前体,内质网工程[69]提高蛋白质的折叠合成和容纳空间,对代谢途径的精准调控都展现了极大的应用潜力。
2.2 合成生物学推进萜类化合物产业化在这些策略的加持下,萜类化合物细胞工厂的建造变得触手可及,但仍需面临一个关键问题:如何从众多通过高效发现手段筛选出的萜类化合物中,挑选出既具备广阔市场前景,又能彰显生物制造成本优势,从而带来显著经济效益的品种?面对这一挑战,既要着眼于实验室内的技术革新,更要深入剖析市场走向与经济可行性。
一种行之有效的策略是,聚焦那些市场需求明确的萜类化合物,通过优化菌株和生产工艺,实现产量的最大化。对于大宗化合物而言,生物制造的单萜在经济效益上或许难以与现有的传统成熟工艺相媲美[147]。然而,在更为复杂的十五碳及以上的萜类化合物领域,生物制造却展现出令人惊喜的成效(表 2)。从倍半萜中的用于香精香料领域的β-法尼烯[57]、具有抗炎舒缓活性的(–)-α-红没药醇,到二萜中龙涎香醚主要合成前体的二萜香紫苏醇[110],再到三萜中生物全合成注射级疫苗佐剂角鲨烯、具有多种药物特性的稀有人参皂苷,以及四萜中的类胡萝卜(如番茄红素、β- 胡萝卜素、虾青素)等,众多萜类化合物已成功打通了从实验室设计到商业化生产的全链条。
另一种富有前瞻性的策略是,通过生物制造满足特定市场需求的全新化合物。在生物制造航空燃料的探索中,研究人员利用细胞工厂快速合成了17个经过理论计算筛选过的倍半萜化合物,并对这些高能液体燃料候选分析进行了系统性的高效评估[56]。这种将细胞工厂与高效发现策略相结合的方法,为拓展到其他细分领域提供了无限可能。
2.3 合成生物学与化学合成的深度融合尽管合成生物学在萜类化合物生物合成方面取得了显著进展,但仍有部分高活性的植物源萜类化合物(如紫杉醇)的生物合成途径难以挖掘,这在一定程度上制约了其产业化进程。然而,随着对生物合成和化学合成认识的不断加深,科学家们开创性地提出了结合两者优势的新策略。
这一策略利用现有的天然产物类似物和最低限度的化学催化,来合成特定的天然产物,有效解决了从复杂天然产物中经济地生产候选药物的难题。美国Amyris公司在酿酒酵母中高产青蒿素前体青蒿酸的实践,就是这一策略的成功典范[100, 148]。遗憾的是,由于市场需求变化等外部因素,Amyris公司的商业化进程遭遇挫折,但这一思路无疑为萜类化合物的产业化开辟了全新路径。
在此基础上,科学家们进一步拓展了那些只停留在高产阶段,而无法进入产业化阶段的化合物的全新运用,实现了其高附加值提升。具有显著抗肾癌活性的Englerin A的大量生产难题,通过微生物合成的愈创木酚-6, 10(14)-二烯作为关键前体实现化学半合成得到解决[149]。具有葡萄柚香味的高附加值产品圆柚酮,利用在酿酒酵母体内高产的倍半萜化合物瓦伦烯一步化学合成[94],实现了产业化的新途径。此外,还能利用类似于化学合成式发散探索的新策略,以生物制造的香叶基香叶醇为出发底物,通过化学合成创制抗溃疡药物替普瑞酮、骨质疏松症治疗药物Menaquinone-4,以及营养品α-生育三烯酚的全新合成途径[150]。
合成生物学在化学半合成领域的应用,不仅实现了复杂天然产物的创制,更能对传统工业生产模式产生颠覆性影响。以维生素E为例,其化学结构中包含萜类结构单元,传统上依赖化学法合成。而现在通过微生物发酵合成的法尼烯作为前体,化学合成获得维生素E的关键中间体异植物醇,并最终合成维生素E[151]。这一创新性技术的突破,打破了国外长期垄断的化学全合成技术,标志着合成生物学在天然产物合成领域的重大进展[152]。
2.4 细胞工厂规模化放大的障碍在萜类化合物的高效发现与产业化制造领域,上游菌株构建技术已实现了丰富的技术储备,并取得了显著进展。然而,在下游环节,特别是规模化生产与分离纯化方面,仍面临重大挑战,成为制约产业发展的瓶颈(图 2)。
(1)规模化放大的困境。从实验室的摇瓶发酵(仅50 mL)到工厂规模的吨级生产,萜类化合物的产业化之路存在大量挑战。这一过程中,需要依次经历实验室小试(约5 L)、中试(接近工业化生产的500 L)等多个环节,直至最终的规模化放大。这些环节中,需要依次摸索最佳的培养基、温度、pH值、溶氧控制、乙醇积累、补料策略等条件,以确保工艺在放大过程中的稳定性和可重复性。然而,发酵罐的实时监测和调控任务繁重且高度依赖工作人员经验。在此背景下,人工智能技术的引入能够加速确认调控操作与菌株代谢特性之间的复杂关系,推进产物合成[153],从而在规模化放大过程中保持产量和质量一致性。
(2)分离纯化的难题。虽然加入有机相进行两相发酵可显著提高产量[154],但易在发酵过程中形成稳定的乳化现象,使有机相和水相分离困难,造成总产量的损失,以及精制困难。开发与发酵过程集成的在线油分离技术,从而允许如细胞重复使用等策略或许能成为破乳的关键。完成破乳后,还需进行纯化。当前,工业上普遍采用高能耗的精馏塔进行分离,但该技术依赖大型设备,与实验室规模脱节[53]。因此,加强科研机构与企业之间的紧密合作,深化产学研融合,成为推动技术创新与产业升级的必然选择。同时,探索更多高效分离策略也显得尤为重要,如超临界流体萃取技术的开发及新型固相萃取试剂的研究等,这些新技术和新方法都有望为萜类化合物的分离纯化提供全新的解决方案。
3 结语随着合成生物学技术的日新月异,萜类化合物这一自然界中极具潜力的化学宝藏,其高效发现与产业化制造正步入一个崭新的发展阶段。在发现方面,创新策略,如高效前体供给底盘开发、基因组与高通量技术协同优化及人工智能应用,加速了萜类化合物的筛选优化,为产业化打下坚实基础。在制造方面,尽管高效细胞工厂的构建已不再成为制约产业发展的瓶颈,但在产物的选择、规模化放大、分离提纯等方面,仍面临诸多挑战。如何进一步优化生产工艺,提高产物纯度与产量,降低成本,是当前亟待解决的问题。
然而,在萜类化合物产业化制造快速发展的同时,也必须清醒地认识到,目前萜类化合物的研究更集中于学术界,众多萜烯的实际应用价值未得到发挥,因此推进其产业化至关重要。为此需要采取一系列措施,以推动学术实现产业转化。①鼓励以市场为导向的应用型科研,建立科研院所与企业的长期合作关系,调整研究方向以满足市场需求,实现资源共享与优势互补。②引入跨学科教育模式,培养既懂科研又懂市场的复合型人才,促进产学研融合,消除科研与市场间的信息障碍。③提供技术和资金支持,制定利于成果转化的政策,规范行业发展。④通过技术交流会、展览、论坛及现代媒体,加大成果推广力度,提升公众认知和市场认可度。
与此同时,对企业而言,萜类化合物的产业化进程仍面临法律法规不完善、知识产权保护不足等挑战。①加快政策革新,推动完善生物制造产品的法律法规,精简商品化审批流程,以促进产业的快速发展,提高企业的创新积极性。②进一步强化知识产权保护体系,加大对技术模仿与侵权行为的打击力度,保护创新者的合法权益,为创新者营造公平、公正的竞争环境。
展望未来,合成生物学技术将持续推动萜类化合物产业化制造迈向更广阔的前景。通过持续的创新突破,将进一步降低成本、提高产量与纯度,满足市场需求,推动产业升级。期待看到更多成功案例的涌现,为生物经济注入新活力,引领科技新时代。
| [1] |
Bureau J A, Oliva M E, Dong Y M, et al. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Natural Product Reports, 2023, 40(12): 1822-1848. DOI:10.1039/D3NP00005B |
| [2] |
奚萌宇, 胡逸灵, 顾玉诚, 等. 基因组挖掘指导天然药物分子的发现. 合成生物学, 2024, 5(3): 447-473. Xi M Y, Hu Y L, Gu Y C, et al. Genome mining-directed discovery for natural medicinal products. Synthetic Biology Journal, 2024, 5(3): 447-473. (in Chinese) |
| [3] |
Atanasov A G, Zotchev S B, Dirsch V M, et al. Natural products in drug discovery: Advances and opportunities. Nature Reviews Drug Discovery, 2021, 20(3): 200-216. DOI:10.1038/s41573-020-00114-z |
| [4] |
胡哲辉, 徐娟, 卞光凯. 自动化高通量技术在天然产物生物合成中的应用. 合成生物学, 2023, 4(5): 932-946. Hu Z H, Xu J, Bian G K. Application of automated highthroughput technology in natural product biosynthesis. Synthetic Biology Journal, 2023, 4(5): 932-946. (in Chinese) |
| [5] |
雷茹, 陶慧, 刘天罡. 基因组深度挖掘驱动微生物萜类化合物高效发现. 合成生物学, 2024, 5(3): 507-526. Lei R, Tao H, Liu T G. Deep genome mining boosts the discovery of microbial terpenoids. Synthetic Biology Journal, 2024, 5(3): 507-526. (in Chinese) |
| [6] |
池豪铭, 刘天罡. 合成生物学助力天然产物的高效合成及创新发现. 生命科学, 2021, 33(12): 1510-1519. Chi H M, Liu T G. Synthetic biology promotes efficient production and innovative discovery of natural products. Chinese Bulletin of Life Sciences, 2021, 33(12): 1510-1519. (in Chinese) |
| [7] |
程术, 邓子新, 卞光凯, 等. 萜类高效合成平台的搭建与萜类产物批量挖掘. 生命科学, 2019, 31(5): 449-457. Cheng S, Deng Z X, Bian G K, et al. Construction of highefficient terpenoid platform and the application in terpenoid discovery. Chinese Bulletin of Life Sciences, 2019, 31(5): 449-457. (in Chinese) |
| [8] |
Zhang J, Hansen L G, Gudich O, et al. A microbial supply chain for production of the anti-cancer drug vinblastine. Nature, 2022, 609: 341-347. DOI:10.1038/s41586-022-05157-3 |
| [9] |
Ro D K, Paradise E M, Ouellet M, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature, 2006, 440: 940-943. DOI:10.1038/nature04640 |
| [10] |
Liu Q, Manzano D, Tanić N, et al. Elucidation and in planta reconstitution of the parthenolide biosynthetic pathway. Metabolic Engineering, 2014, 23: 145-153. DOI:10.1016/j.ymben.2014.03.005 |
| [11] |
Li C F, Li Y J, Wang J X, et al. An independent biosynthetic route to frame a xanthanolide-type sesquiterpene lactone in Asteraceae. The Plant Journal: For Cell and Molecular Biology, 2024. DOI:10.1111/tpj.17199 |
| [12] |
Han L Y, Wu Y K, Xu Y M, et al. Engineered Saccharomyces cerevisiae for de novo δ-tocotrienol biosynthesis. Systems Microbiology and Biomanufacturing, 2024, 4(1): 150-164. DOI:10.1007/s43393-023-00167-2 |
| [13] |
Hansen N L, Kjaerulff L, Heck Q K, et al. Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide. Nature Communications, 2022, 13(1): 5011-5022. DOI:10.1038/s41467-022-32667-5 |
| [14] |
Jiang B, Gao L, Wang H J, et al. Characterization and heterologous reconstitution of Taxus biosynthetic enzymes leading to baccatin Ⅲ. Science, 2024, 383: 622-629. DOI:10.1126/science.adj3484 |
| [15] |
Xu R, Zou Y. Biosynthesis of (-)-vinigrol. Angewandte Chemie International Edition, 2024, e202416795. |
| [16] |
Yuan Y J, Cheng S, Bian G K, et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi. Nature Catalysis, 2022, 5: 277-287. DOI:10.1038/s41929-022-00762-x |
| [17] |
Zhang C Z, Wu J, Sun Q, et al. De novo production of bioactive sesterterpenoid ophiobolins in Saccharomyces cerevisiae cell factories. Microbial Cell Factories, 2024, 23(1): 129-142. DOI:10.1186/s12934-024-02406-0 |
| [18] |
De La Peña R, Hodgson H, Liu J C, et al. Complex scaffold remodeling in plant triterpene biosynthesis. Science, 2023, 379: 361-368. DOI:10.1126/science.adf1017 |
| [19] |
Liu Y Z, Zhao X X, Gan F, et al. Complete biosynthesis of QS-21 in engineered yeast. Nature, 2024, 629: 937-944. DOI:10.1038/s41586-024-07345-9 |
| [20] |
Yuan W, Jiang C J, Wang Q, et al. Biosynthesis of mushroomderived type Ⅱ ganoderic acids by engineered yeast. Nature Communications, 2022, 13(1): 7715-7740. DOI:10.1038/s41467-022-35310-5 |
| [21] |
Liao J J, Liu T Y, Xie L, et al. Heterologous mogrosides biosynthesis in cucumber and tomato by genetic manipulation. Communications Biology, 2023, 6(1): 191-202. DOI:10.1038/s42003-023-04553-3 |
| [22] |
Cao Z Q, Li S Y, Lv J M, et al. Biosynthesis of clinically used antibiotic fusidic acid and identification of two short-chain dehydrogenase/reductases with converse stereoselectivity. Acta Pharmaceutica Sinica B, 2019, 9(2): 433-442. DOI:10.1016/j.apsb.2018.10.007 |
| [23] |
Cao Z Q, Lv J M, Liu Q, et al. Biosynthetic study of cephalosporin P1 reveals a multifunctional P450 enzyme and a site-selective acetyltransferase. ACS Chemical Biology, 2020, 15(1): 44-51. DOI:10.1021/acschembio.9b00863 |
| [24] |
Xu B Y, Huang J P, Peng G Q, et al. Total biosynthesis of the medicinal triterpenoid saponin astragalosides. Nature Plants, 2024, 10(11): 1826-1837. DOI:10.1038/s41477-024-01827-4 |
| [25] |
Zhu F Y, Zhong X F, Hu M, et al. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnology and Bioengineering, 2014, 111(7): 1396-1405. DOI:10.1002/bit.25198 |
| [26] |
Bian G K, Han Y C, Hou A W, et al. Releasing the potential power of terpene synthases by a robust precursor supply platform. Metabolic Engineering, 2017, 42: 1-8. DOI:10.1016/j.ymben.2017.04.006 |
| [27] |
Lian J Z, HamediRad M, Zhao H M. Advancing metabolic engineering of Saccharomyces cerevisiae using the CRISPR/Cas system. Biotechnology Journal, 2018, 13(9): e1700601. DOI:10.1002/biot.201700601 |
| [28] |
Chen R, Jia Q D, Mu X, et al. Systematic mining of fungal chimeric terpene synthases using an efficient precursorproviding yeast chassis. PNAS, 2021, 118(29): e2023247118. DOI:10.1073/pnas.2023247118 |
| [29] |
Tao H, Lauterbach L, Bian G K, et al. Discovery of nonsqualene triterpenes. Nature, 2022, 606: 414-419. DOI:10.1038/s41586-022-04773-3 |
| [30] |
Zhi Y, Dai C, Fang X T, et al. Gene-directed in vitro mining uncovers the insect-repellent constituent from mugwort (Artemisia argyi). Journal of the American Chemical Society, 2024, 146(45): 30883-30892. DOI:10.1021/jacs.4c08857 |
| [31] |
Bian G K, Deng Z X, Liu T G. Strategies for terpenoid overproduction and new terpenoid discovery. Current Opinion in Biotechnology, 2017, 48: 234-241. DOI:10.1016/j.copbio.2017.07.002 |
| [32] |
Jia X, Song J Y, Wu Y J, et al. Strategies for the enhancement of secondary metabolite production via biosynthesis gene cluster regulation in Aspergillus oryzae. Journal of Fungi, 2024, 10(5): 312. DOI:10.3390/jof10050312 |
| [33] |
肖丽萍, 邓子新, 刘天罡. 链霉菌底盘细胞的开发现状及其应用. 微生物学报, 2016, 56(3): 441-453. Xiao L P, Deng Z X, Liu T G. Progress in developing and applying Streptomyces chassis—A review. Acta Microbiologica Sinica, 2016, 56(3): 441-453. (in Chinese) |
| [34] |
Komatsua M, Uchiyama T, Omura S, et al. Genomeminimized Streptomyces host for the heterologous expression of secondary metabolism. PNAS, 2010, 107(6): 2646-2651. DOI:10.1073/pnas.0914833107 |
| [35] |
Komatsu M, Komatsu K, Koiwai H, et al. Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synthetic Biology, 2013, 2(7): 384-396. DOI:10.1021/sb3001003 |
| [36] |
Yamada Y, Arima S, Nagamitsu T, et al. Novel terpenes generated by heterologous expression of bacterial terpene synthase genes in an engineered Streptomyces host. The Journal of Antibiotics, 2015, 68(6): 385-394. DOI:10.1038/ja.2014.171 |
| [37] |
Lin J J, Yin X, Zeng Y R, et al. Progress and prospect: Biosynthesis of plant natural products based on plant chassis. Biotechnology Advances, 2023, 69: 108266. DOI:10.1016/j.biotechadv.2023.108266 |
| [38] |
邵洁, 李建华, 王凯博, 等. 植物底盘: 天然产物合成生物学研究的新热点. 生物加工过程, 2017, 15(5): 24-31. Shao J, Li J H, Wang K B, et al. Plant chassis: New hotspots of natural product synthetic biology. Chinese Journal of Bioprocess Engineering, 2017, 15(5): 24-31. (in Chinese) |
| [39] |
Kalkreuter E, Pan G H, Cepeda A J, et al. Targeting bacterial genomes for natural product discovery. Trends in Pharmacological Sciences, 2020, 41(1): 13-26. DOI:10.1016/j.tips.2019.11.002 |
| [40] |
涂然, 毛雨丰, 刘叶, 等. 工程菌种自动化高通量编辑与筛选研究进展. 生物工程学报, 2022, 38(11): 4162-4179. Tu R, Mao Y F, Liu Y, et al. Advances in automated highthroughput editing and screening of engineered strains. Chinese Journal of Biotechnology, 2022, 38(11): 4162-4179. (in Chinese) |
| [41] |
崔金明, 张炳照, 马迎飞, 等. 合成生物学研究的工程化平台. 中国科学院院刊, 2018, 33(11): 1249-1257. Cui J M, Zhang B Z, Ma Y F, et al. Engineering platforms for synthetic biology research. Bulletin of Chinese Academy of Sciences, 2018, 33(11): 1249-1257. (in Chinese) |
| [42] |
张亭, 冷梦甜, 金帆, 等. 合成生物研究重大科技基础设施概述. 合成生物学, 2022, 3(1): 184-194. Zhang T, Leng M T, Jin F, et al. Overview on platform for synthetic biology research at Shenzhen. Synthetic Biology Journal, 2022, 3(1): 184-194. (in Chinese) |
| [43] |
Mullowney M W, Duncan K R, Elsayed S S, et al. Artificial intelligence for natural product drug discovery. Nature Reviews Drug Discovery, 2023, 22(11): 895-916. DOI:10.1038/s41573-023-00774-7 |
| [44] |
Saldívar-González F I, Aldas-Bulos V D, Medina-Franco J L, et al. Natural product drug discovery in the artificial intelligence era. Chemical Science, 2021, 13(6): 1526-1546. |
| [45] |
Moore S J, MacDonald J T, Wienecke S, et al. Rapid acquisition and model-based analysis of cell-free transcription – translation reactions from nonmodel bacteria. PNAS, 2018, 115(19): E4340-E4349. |
| [46] |
Liu J Y, Nothias L F, Dorrestein P C, et al. Genomic and metabolomic analysis of the potato common scab pathogen Streptomyces scabiei. ACS Omega, 2021, 6(17): 11474-11487. DOI:10.1021/acsomega.1c00526 |
| [47] |
Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature, 2021, 596: 583-589. DOI:10.1038/s41586-021-03819-2 |
| [48] |
Abramson J, Adler J, Dunger J, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature, 2024, 630: 493-500. DOI:10.1038/s41586-024-07487-w |
| [49] |
杨朔, 王洁, 张梦婷, 等. 基于人工智能的药物-靶标相互作用预测. 中国现代应用药学, 2022, 39(21): 2797-2803. Yang S, Wang J, Zhang M T, et al. Drug-target interaction prediction with artificial intelligence. Chinese Journal of Modern Applied Pharmacy, 2022, 39(21): 2797-2803. (in Chinese) |
| [50] |
Rehman A U, Li M Y, Wu B J, et al. Role of artificial intelligence in revolutionizing drug discovery. Fundamental Research, 2024. DOI:10.1016/j.fmre.2024.04.021 |
| [51] |
刘润哲, 宋俊科, 刘艾林, 等. 人工智能在基于配体和受体结构的药物筛选中的应用进展. 药学学报, 2021, 56(8): 2136-2145. Liu R Z, Song J K, Liu A L, et al. Progress on the application of artificial intelligence technology in ligand-based and receptor structure-based drug screening. Acta Pharmaceutica Sinica, 2021, 56(8): 2136-2145. (in Chinese) |
| [52] |
黄芳, 杨红飞, 朱迅. 人工智能在新药发现中的应用进展. 药学进展, 2021, 45(7): 502-511. Huang F, Yang H F, Zhu X. Progress in the application of artificial intelligence in new drug discovery. Progress in Pharmaceutical Sciences, 2021, 45(7): 502-511. (in Chinese) |
| [53] |
Li T, Liu X M, Xiang H Y, et al. Two-phase fermentation systems for microbial production of plant-derived terpenes. Molecules, 2024, 29(5): 1127. DOI:10.3390/molecules29051127 |
| [54] |
Rinaldi M A, Ferraz C A, Scrutton N S. Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli. Natural Product Reports, 2022, 39(1): 90-118. DOI:10.1039/D1NP00025J |
| [55] |
Gao J Q, Li Y X, Yu W, et al. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol. Nature Metabolism, 2022, 4(7): 932-943. DOI:10.1038/s42255-022-00601-0 |
| [56] |
Huang Y L, Ye Z L, Wan X K, et al. Systematic mining and evaluation of the sesquiterpene skeletons as high energy aviation fuel molecules. Advanced Science, 2023, 10(23): e2300889. DOI:10.1002/advs.202300889 |
| [57] |
Meadows A L, Hawkins K M, Tsegaye Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature, 2016, 537: 694-697. DOI:10.1038/nature19769 |
| [58] |
Ma Y S, Zu Y X, Huang S W, et al. Engineering a universal and efficient platform for terpenoid synthesis in yeast. PNAS, 2023, 120(1): e2207680120. DOI:10.1073/pnas.2207680120 |
| [59] |
Ma B, Liu M, Li Z H, et al. Significantly enhanced production of patchoulol in metabolically engineered Saccharomyces cerevisiae. Journal of Agricultural and Food Chemistry, 2019, 67(31): 8590-8598. DOI:10.1021/acs.jafc.9b03456 |
| [60] |
Hong J, Park S H, Kim S, et al. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Applied Microbiology and Biotechnology, 2019, 103(1): 211-223. DOI:10.1007/s00253-018-9449-8 |
| [61] |
Chen R B, Gao J Q, Yu W, et al. Engineering cofactor supply and recycling to drive phenolic acid biosynthesis in yeast. Nature Chemical Biology, 2022, 18(5): 520-529. DOI:10.1038/s41589-022-01014-6 |
| [62] |
Amna B, Su L Q, Dai Z J, et al. Enzyme engineering in microbial biosynthesis of terpenoids: Progress and perspectives. Sheng Wu Gong Cheng Xue Bao, 2024, 40(8): 2473-2488. |
| [63] |
Kang W, Ma T, Liu M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux. Nature Communications, 2019, 10(1): 4248-4258. DOI:10.1038/s41467-019-12247-w |
| [64] |
Sun X X, Yuan Y J, Chen Q T, et al. Metabolic pathway assembly using docking domains from type Ⅰ Cis-AT polyketide synthases. Nature Communications, 2022, 13(1): 5541-5552. DOI:10.1038/s41467-022-33272-2 |
| [65] |
Zhu K, Kong J, Zhao B X, et al. Metabolic engineering of microbes for monoterpenoid production. Biotechnology Advances, 2021, 53: 107837. DOI:10.1016/j.biotechadv.2021.107837 |
| [66] |
Zhu Z T, Du M M, Gao B, et al. Metabolic compartmentalization in yeast mitochondria: Burden and solution for squalene overproduction. Metabolic Engineering, 2021, 68: 232-245. DOI:10.1016/j.ymben.2021.10.011 |
| [67] |
Ma Y S, Shang Y, Stephanopoulos G. Engineering peroxisomal biosynthetic pathways for maximization of triterpene production in Yarrowia lipolytica. PNAS, 2024, 121(5): e2314798121. DOI:10.1073/pnas.2314798121 |
| [68] |
Ma T, Shi B, Ye Z L, et al. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metabolic Engineering, 2019, 52: 134-142. DOI:10.1016/j.ymben.2018.11.009 |
| [69] |
Shi Y T, Dong T Y, Zeng B X, et al. Production of plant sesquiterpene lactone parthenolide in the yeast cell factory. ACS Synthetic Biology, 2022, 11(7): 2473-2483. DOI:10.1021/acssynbio.2c00132 |
| [70] |
Wang X, Xiao L J, Zhang X Y, et al. Combined bioderivatization and engineering approach to improve the efficiency of geraniol production. Green Chemistry, 2022, 24(2): 864-876. DOI:10.1039/D1GC03419G |
| [71] |
Baker J J, Shi J, Wang S Y, et al. ML-enhanced peroxisome capacity enables compartmentalization of multienzyme pathway. Nature Chemical Biology, 2024. DOI:10.1038/s41589-024-01759-2 |
| [72] |
Zhao C, Wang X H, Lu X Y, et al. Spatiotemporal regulation and transport engineering for sustainable production of geraniol in Candida glycerinogenes. Journal of Agricultural and Food Chemistry, 2024, 72(9): 4825-4833. DOI:10.1021/acs.jafc.3c09651 |
| [73] |
Agrawal A, Yang Z L, Blenner M. Engineering Yarrowia lipolytica for the biosynthesis of geraniol. Metabolic Engineering Communications, 2023, 17: e00228. DOI:10.1016/j.mec.2023.e00228 |
| [74] |
Jiang G Z, Yao M D, Wang Y, et al. A "push-pull-restrain" strategy to improve citronellol production in Saccharomyces cerevisiae. Metabolic Engineering, 2021, 66: 51-59. DOI:10.1016/j.ymben.2021.03.019 |
| [75] |
Zhou P P, Zhou X Q, Yuan D D, et al. Combining protein and organelle engineering for linalool overproduction in Saccharomyces cerevisiae. Journal of Agricultural and Food Chemistry, 2023, 71(26): 10133-10143. DOI:10.1021/acs.jafc.2c08416 |
| [76] |
Hoshino Y, Moriya M, Matsudaira A, et al. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. Journal of Biotechnology, 2020, 324: 21-27. DOI:10.1016/j.jbiotec.2020.09.021 |
| [77] |
Wu J, Wang X, Xiao L J, et al. Synthetic protein scaffolds for improving R-(-)-linalool production in Escherichia coli. Journal of Agricultural and Food Chemistry, 2021, 69(20): 5663-5670. DOI:10.1021/acs.jafc.1c01101 |
| [78] |
Rolf J, Julsing M K, Rosenthal K, et al. A gram-scale limonene production process with engineered Escherichia coli. Molecules, 2020, 25(8): 1881. DOI:10.3390/molecules25081881 |
| [79] |
Kong X, Wu Y K, Yu W W, et al. Efficient synthesis of limonene in Saccharomyces cerevisiae using combinatorial metabolic engineering strategies. Journal of Agricultural and Food Chemistry, 2023, 71(20): 7752-7764. DOI:10.1021/acs.jafc.3c02076 |
| [80] |
Gao Q D, Dong Y Q, Huang Y, et al. Dual-regulation in peroxisome and cytoplasm toward efficient limonene biosynthesis with Rhodotorula toruloides. ACS Synthetic Biology, 2024, 13(8): 2545-2554. DOI:10.1021/acssynbio.4c00306 |
| [81] |
Zhang H B, Liu Q, Cao Y J, et al. Microbial production of sabinene—A new terpene-based precursor of advanced biofuel. Microbial Cell Factories, 2014, 13: 20-29. DOI:10.1186/1475-2859-13-20 |
| [82] |
Ye C F, Li M X, Gao J C, et al. Metabolic engineering of Pichia pastoris for overproduction of Cis-trans nepetalactol. Metabolic Engineering, 2024, 84: 83-94. DOI:10.1016/j.ymben.2024.06.007 |
| [83] |
Kirby J, Geiselman G M, Yaegashi J, et al. Further engineering of R. toruloides for the production of terpenes from lignocellulosic biomass. Biotechnology for Biofuels, 2021, 14(1): 101-116. DOI:10.1186/s13068-021-01950-w |
| [84] |
Liu Y H, Jiang X, Cui Z Y, et al. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene. Biotechnology for Biofuels, 2019, 12: 296-303. DOI:10.1186/s13068-019-1636-z |
| [85] |
Liu Y H, Zhang J, Li Q B, et al. Engineering Yarrowia lipolytica for the sustainable production of β-farnesene from waste oil feedstock. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 101-115. DOI:10.1186/s13068-022-02201-2 |
| [86] |
Tan N, Ong L, Shukal S, et al. High-yield biosynthesis of trans-nerolidol from sugar and glycerol. Journal of Agricultural and Food Chemistry, 2023, 71(22): 8479-8487. DOI:10.1021/acs.jafc.3c01161 |
| [87] |
Liu F, Liu S C, Qi Y K, et al. Enhancing Trans-nerolidol productivity in Yarrowia lipolytica by improving precursor supply and optimizing nerolidol synthase activity. Journal of Agricultural and Food Chemistry, 2022, 70(48): 15157-15165. DOI:10.1021/acs.jafc.2c05847 |
| [88] |
Zhao B X, Zhang Y H, Wang Y P, et al. Biosynthesis of α-bisabolene from low-cost renewable feedstocks by peroxisome engineering and systems metabolic engineering of the yeast Yarrowia lipolytica. Green Chemistry, 2023, 25(20): 8145-8159. DOI:10.1039/D3GC01936E |
| [89] |
Zhang Y, Song X H, Lai Y M, et al. High-yielding terpene— Based biofuel production in Rhodobacter capsulatus. ACS Synthetic Biology, 2021, 10(6): 1545-1552. DOI:10.1021/acssynbio.1c00146 |
| [90] |
Lu Y, Liu D, Wang L, et al. Constructing high-yielding Serratia marcescens for (-)-α-bisabolol production based on the exogenous haloarchaeal MVA pathway and endogenous molecular chaperones. Journal of Agricultural and Food Chemistry, 2025, 73(1): 747-755. DOI:10.1021/acs.jafc.4c10135 |
| [91] |
Lim H S, Kim S K, Woo S G, et al. (–)-α-Bisabolol production in engineered Escherichia coli expressing a novel (–)-α-Bisabolol synthase from the globe artichoke Cynara cardunculus var. Scolymus. Journal of Agricultural and Food Chemistry, 2021, 69(30): 8492-8503. DOI:10.1021/acs.jafc.1c02759 |
| [92] |
Jiang Y K, Xia L, Gao S, et al. Engineering Saccharomyces cerevisiae for enhanced (–)-α-bisabolol production. Synthetic and Systems Biotechnology, 2023, 8(2): 187-195. DOI:10.1016/j.synbio.2023.01.004 |
| [93] |
Zuo Y M, Xiao F, Gao J C, et al. Establishing Komagataella phaffii as a cell factory for efficient production of sesquiterpenoid α-santalene. Journal of Agricultural and Food Chemistry, 2022, 70(26): 8024-8031. DOI:10.1021/acs.jafc.2c02353 |
| [94] |
Ye Z L, Huang Y L, Shi B, et al. Coupling cell growth and biochemical pathway induction in Saccharomyces cerevisiae for production of (+)-valencene and its chemical conversion to (+)-nootkatone. Metabolic Engineering, 2022, 72: 107-115. DOI:10.1016/j.ymben.2022.03.005 |
| [95] |
Deng X M, Shi B, Ye Z L, et al. Systematic identification of Ocimum sanctum sesquiterpenoid synthases and (–)-eremophilene overproduction in engineered yeast. Metabolic Engineering, 2022, 69: 122-133. DOI:10.1016/j.ymben.2021.11.005 |
| [96] |
Liu Q, Zhang G, Su L Q, et al. Reprogramming the metabolism of oleaginous yeast for sustainably biosynthesizing the anticarcinogen precursor germacrene A. Green Chemistry, 2023, 25(20): 7988-7997. DOI:10.1039/D3GC01661G |
| [97] |
Liu J J, Chen C, Wan X K, et al. Identification of the sesquiterpene synthase AcTPS1 and high production of (-)-germacrene D in metabolically engineered Saccharomyces cerevisiae. Microbial Cell Factories, 2022, 21(1): 89-102. DOI:10.1186/s12934-022-01814-4 |
| [98] |
Zhou L, Wang Q, Shen J W, et al. Metabolic engineering of glycolysis in Escherichia coli for efficient production of patchoulol and τ-cadinol. Bioresource Technology, 2024, 391: 130004. DOI:10.1016/j.biortech.2023.130004 |
| [99] |
Westfall P J, Pitera D J, Lenihan J R, et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. PNAS, 2012, 109(3): E111-E118. |
| [100] |
Paddon C J, Westfall P J, Pitera D J, et al. High-level semisynthetic production of the potent antimalarial artemisinin. Nature, 2013, 496: 528-532. DOI:10.1038/nature12051 |
| [101] |
Guo Q, Li Y W, Yan F, et al. Dual cytoplasmic-peroxisomal engineering for high-yield production of sesquiterpene α-humulene in Yarrowia lipolytica. Biotechnology and Bioengineering, 2022, 119(10): 2819-2830. DOI:10.1002/bit.28176 |
| [102] |
Zhang C B, Chen C, Bian X K, et al. Construction of an orthogonal transport system for Saccharomyces cerevisiae peroxisome to efficiently produce sesquiterpenes. Metabolic Engineering, 2024, 85: 84-93. DOI:10.1016/j.ymben.2024.07.010 |
| [103] |
Li Z P, Gan Y H, Gou C Y, et al. Efficient biosynthesis of β-caryophyllene in Saccharomyces cerevisiae by β-caryophyllene synthase from Artemisia argyi. Synthetic and Systems Biotechnology, 2025, 10(1): 158-164. DOI:10.1016/j.synbio.2024.09.005 |
| [104] |
Cheng T, Zhang K, Guo J, et al. Highly efficient biosynthesis of β-caryophyllene with a new sesquiterpene synthase from tobacco. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 39-52. DOI:10.1186/s13068-022-02136-8 |
| [105] |
Shukal S, Chen X X, Zhang C Q. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metabolic Engineering, 2019, 55: 170-178. DOI:10.1016/j.ymben.2019.07.007 |
| [106] |
Wang J H, Li Y R, Jiang W, et al. Engineering Saccharomyces cerevisiae YPH499 for overproduction of geranylgeraniol. Journal of Agricultural and Food Chemistry, 2023, 71(25): 9804-9814. DOI:10.1021/acs.jafc.3c01820 |
| [107] |
Wang K F, Yin M X, Sun M L, et al. Engineering Yarrowia lipolytica for efficient synthesis of geranylgeraniol. Journal of Agricultural and Food Chemistry, 2024, 72(37): 20568-20581. DOI:10.1021/acs.jafc.4c06749 |
| [108] |
Zhang L H, Fan C, Yang H Q, et al. Biosynthetic pathway redesign in non-conventional yeast for enhanced production of cembratriene-ol. Bioresource Technology, 2024, 399: 130596. DOI:10.1016/j.biortech.2024.130596 |
| [109] |
Ajikumar P K, Xiao W H, Tyo K E J, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science, 2010, 330: 70-74. DOI:10.1126/science.1191652 |
| [110] |
Cao X, Yu W, Chen Y, et al. Engineering yeast for highlevel production of diterpenoid sclareol. Metabolic Engineering, 2023, 75: 19-28. DOI:10.1016/j.ymben.2022.11.002 |
| [111] |
Hu T Y, Zhou J W, Tong Y R, et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metabolic Engineering, 2020, 60: 87-96. DOI:10.1016/j.ymben.2020.03.011 |
| [112] |
Zhang C B, Ju H Y, Lu C Z, et al. High-titer production of 13R-manoyl oxide in metabolically engineered Saccharomyces cerevisiae. Microbial Cell Factories, 2019, 18(1): 73-81. DOI:10.1186/s12934-019-1123-z |
| [113] |
Geiselman G M, Zhuang X, Kirby J, et al. Production of ent-kaurene from lignocellulosic hydrolysate in Rhodosporidium toruloides. Microbial Cell Factories, 2020, 19(1): 24-35. DOI:10.1186/s12934-020-1293-8 |
| [114] |
Sun Y W, Chen Z, Wang G Y, et al. De novo production of versatile oxidized kaurene diterpenes in Escherichia coli. Metabolic Engineering, 2022, 73: 201-213. DOI:10.1016/j.ymben.2022.08.001 |
| [115] |
Xu Y M, Wang X L, Zhang C Y, et al. De novo biosynthesis of rubusoside and rebaudiosides in engineered yeasts. Nature Communications, 2022, 13(1): 3040-3051. DOI:10.1038/s41467-022-30826-2 |
| [116] |
Xiao M L, Wang Y M, Wang Y, et al. Repurposing the cellulase workhorse Trichoderma reesei as a ROBUST chassis for efficient terpene production. Green Chemistry, 2023, 25(18): 7362-7371. DOI:10.1039/D3GC01770B |
| [117] |
Ning Y, Liu M S, Ru Z Y, et al. Efficient synthesis of squalene by cytoplasmic-peroxisomal engineering and regulating lipid metabolism in Yarrowia lipolytica. Bioresource Technology, 2024, 395: 130379. DOI:10.1016/j.biortech.2024.130379 |
| [118] |
Park J, Kang D H, Woo H M. Microbial bioprocess for extracellular squalene production and formulation of nanoemulsions. ACS Sustainable Chemistry & Engineering, 2021, 9(42): 14263-14276. |
| [119] |
Jia N, Li J Z, Zang G W, et al. Engineering Saccharomyces cerevisiae for high-efficient production of ursolic acid via cofactor engineering and acetyl-CoA optimization. Biochemical Engineering Journal, 2024, 203: 109189. DOI:10.1016/j.bej.2023.109189 |
| [120] |
Du M M, Zhang G G, Zhu Z T, et al. Boosting the epoxidation of squalene to produce triterpenoids in Saccharomyces cerevisiae. Biotechnology for Biofuels and Bioproducts, 2023, 16(1): 76-90. DOI:10.1186/s13068-023-02310-6 |
| [121] |
Sun W T, Wan S T, Liu C Y, et al. Establishing cell suitability for high-level production of licorice triterpenoids in yeast. Acta Pharmaceutica Sinica B, 2024, 14(9): 4134-4148. DOI:10.1016/j.apsb.2024.04.032 |
| [122] |
Cheng X, Pang Y R, Ban Y L, et al. Application of multiple strategies to enhance oleanolic acid biosynthesis by engineered Saccharomyces cerevisiae. Bioresource Technology, 2024, 401: 130716. DOI:10.1016/j.biortech.2024.130716 |
| [123] |
Huang J J, Zha W L, An T Y, et al. Identification of RoCYP01 (CYP716A155) enables construction of engineered yeast for high-yield production of betulinic acid. Applied Microbiology and Biotechnology, 2019, 103(17): 7029-7039. DOI:10.1007/s00253-019-10004-z |
| [124] |
Dong T Y, Zhou X, Hou Z J, et al. Multiple strategies enhance 7-dehydrocholesterol production from kitchen waste by engineered Yarrowia lipolytica. Journal of Agricultural and Food Chemistry, 2025, 73(1): 693-705. DOI:10.1021/acs.jafc.4c09552 |
| [125] |
Yin X R, Wei W Q, Chen Q H, et al. Reengineering the substrate tunnel to enhance the catalytic efficiency of squalene epoxidase. Journal of Agricultural and Food Chemistry, 2024, 72(44): 24599-24608. DOI:10.1021/acs.jafc.4c05892 |
| [126] |
Yin X R, Zhang Y L, Wei W Q, et al. Overproduction of cucurbitadienol through modular metabolic engineering and fermentation optimization in Saccharomyces cerevisiae. Journal of Agricultural and Food Chemistry, 2025, 73(1): 718-726. DOI:10.1021/acs.jafc.4c09684 |
| [127] |
Gan Y H, Li Z P, Fan B L, et al. De novo biosynthesis of a polyene-type ginsenoside precursor dammaradienol in Saccharomyces cerevisiae. ACS Synthetic Biology, 2024, 13(12): 4015-4026. DOI:10.1021/acssynbio.4c00396 |
| [128] |
王冬, 刘怡, 许骄阳, 等. 创建酿酒酵母细胞工厂高效生产人参皂苷前体达玛烯二醇Ⅱ. 药学学报, 2018, 53(8): 1233-1241. Wang D, Liu Y, Xu J Y, et al. Construction of efficient yeast cell factories for production of ginsenosides precursor dammarenediol-Ⅱ. Acta Pharmaceutica Sinica, 2018, 53(8): 1233-1241. (in Chinese) |
| [129] |
Pang Y R, Cheng X, Ban Y L, et al. Efficient production of 22(R)-hydroxycholesterol via combination optimization of Saccharomyces cerevisiae. Biotechnology Journal, 2024, 19(7): e2400286. DOI:10.1002/biot.202400286 |
| [130] |
Zhu Y, Li J X, Peng L Y, et al. High-yield production of protopanaxadiol from sugarcane molasses by metabolically engineered Saccharomyces cerevisiae. Microbial Cell Factories, 2022, 21(1): 230-242. DOI:10.1186/s12934-022-01949-4 |
| [131] |
Li X D, Wang Y M, Fan Z J, et al. High-level sustainable production of the characteristic protopanaxatriol-type saponins from Panax species in engineered Saccharomyces cerevisiae. Metabolic Engineering, 2021, 66: 87-97. DOI:10.1016/j.ymben.2021.04.006 |
| [132] |
Wang P P, Wei W, Ye W, et al. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at highefficiency. Cell Discovery, 2019, 5: 5-18. DOI:10.1038/s41421-018-0075-5 |
| [133] |
Li C J, Yan X, Xu Z Z, et al. Pathway elucidation of bioactive rhamnosylated ginsenosides in Panax ginseng and their de novo high-level production by engineered Saccharomyces cerevisiae. Communications Biology, 2022, 5(1): 775-783. DOI:10.1038/s42003-022-03740-y |
| [134] |
Wang P P, Wang J L, Zhao G P, et al. Systematic optimization of the yeast cell factory for sustainable and high efficiency production of bioactive ginsenoside compound K. Synthetic and Systems Biotechnology, 2021, 6(2): 69-76. DOI:10.1016/j.synbio.2021.03.002 |
| [135] |
Hu Z F, Gu A D, Liang L, et al. Construction and optimization of microbial cell factories for sustainable production of bioactive dammarenediol-Ⅱ glucosides. Green Chemistry, 2019, 21(12): 3286-3299. DOI:10.1039/C8GC04066D |
| [136] |
Park K, Kim G, Cha S, et al. Efficient production of the colorless carotenoid phytoene in Yarrowia lipolytica through metabolic engineering. Journal of Agricultural and Food Chemistry, 2024, 72(48): 26786-26795. DOI:10.1021/acs.jafc.4c07735 |
| [137] |
Ma Y S, Liu N, Greisen P, et al. Removal of lycopene substrate inhibition enables high carotenoid productivity in Yarrowia lipolytica. Nature Communications, 2022, 13(1): 572-582. DOI:10.1038/s41467-022-28277-w |
| [138] |
Zhou K, Yu C, Liang N, et al. Adaptive evolution and metabolic engineering boost lycopene production in Saccharomyces cerevisiae via enhanced precursors supply and utilization. Journal of Agricultural and Food Chemistry, 2023, 71(8): 3821-3831. DOI:10.1021/acs.jafc.2c08579 |
| [139] |
Sun T, Miao L T, Li Q Y, et al. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnology Letters, 2014, 36(7): 1515-1522. DOI:10.1007/s10529-014-1543-0 |
| [140] |
Li K, Li C, Liu C G, et al. Engineering carbon source division of labor for efficient α-carotene production in Corynebacterium glutamicum. Metabolic Engineering, 2024, 84: 117-127. DOI:10.1016/j.ymben.2024.06.008 |
| [141] |
Wu Y Q, Yan P P, Li Y, et al. Enhancing β-carotene production in Escherichia coli by perturbing central carbon metabolism and improving the NADPH supply. Frontiers in Bioengineering and Biotechnology, 2020, 8: 585-597. DOI:10.3389/fbioe.2020.00585 |
| [142] |
Chen M K, Li M, Ye L D, et al. Construction of canthaxanthin-producing yeast by combining spatiotemporal regulation and pleiotropic drug resistance engineering. ACS Synthetic Biology, 2022, 11(1): 325-333. DOI:10.1021/acssynbio.1c00437 |
| [143] |
Zhu H Z, Jiang S, Wu J J, et al. Production of high levels of 3 S, 3' S-astaxanthin in Yarrowia lipolytica via iterative metabolic engineering. Journal of Agricultural and Food Chemistry, 2022, 70(8): 2673-2683. DOI:10.1021/acs.jafc.1c08072 |
| [144] |
Ren X F, Liu M S, Yue M Y, et al. Metabolic pathway coupled with fermentation process optimization for highlevel production of retinol in Yarrowia lipolytica. Journal of Agricultural and Food Chemistry, 2024, 72(15): 8664-8673. DOI:10.1021/acs.jafc.4c00377 |
| [145] |
Shi Y, Lu S H, Zhou X, et al. Systematic metabolic engineering enables highly efficient production of vitamin A in Saccharomyces cerevisiae. Synthetic and Systems Biotechnology, 2025, 10(1): 58-67. DOI:10.1016/j.synbio.2024.08.004 |
| [146] |
Lin J Y, Bu X, Lan Y B, et al. Combined metabolic engineering and lipid droplets degradation to increase vitamin A production in Saccharomyces cerevisiae. Microbial Cell Factories, 2024, 23(1): 317-328. DOI:10.1186/s12934-024-02596-7 |
| [147] |
Wu W Z, Maravelias C T. Synthesis and techno-economic assessment of microbial-based processes for terpenes production. Biotechnology for Biofuels, 2018, 11: 294-307. DOI:10.1186/s13068-018-1285-7 |
| [148] |
Peplow M. Synthetic biology's first malaria drug meets market resistance. Nature, 2016, 530: 389-390. DOI:10.1038/530390a |
| [149] |
Siemon T, Wang Z Q, Bian G K, et al. Semisynthesis of plant-derived englerin A enabled by microbe engineering of guaia-6, 10(14)-diene as building block. Journal of the American Chemical Society, 2020, 142(6): 2760-2765. DOI:10.1021/jacs.9b12940 |
| [150] |
Chi H M, Wen S, Wen T, et al. Geranylgeraniol: Bio-based platform for teprenone, menaquinone-4, and α-tocotrienol synthesis. Bioresource Technology, 2024, 411: 131349. DOI:10.1016/j.biortech.2024.131349 |
| [151] |
Ye Z L, Shi B, Huang Y L, et al. Revolution of vitamin E production by starting from microbial fermented farnesene to isophytol. The Innovation, 2022, 3(3): 100228. DOI:10.1016/j.xinn.2022.100228 |
| [152] |
马田, 邓子新, 刘天罡. 维生素E的"前世"和"今生". 合成生物学, 2020, 1(2): 174-186. Ma T, Deng Z X, Liu T G. The past and present of vitamin E. Synthetic Biology Journal, 2020, 1(2): 174-186. (in Chinese) |
| [153] |
夏建业, 刘晶, 庄英萍. 人工智能时代发酵优化与放大技术的机遇与挑战. 生物工程学报, 2022, 38(11): 4180-4199. Xia J Y, Liu J, Zhuang Y P. Opportunities and challenges for fermentation optimization and scale-up technology in the artificial intelligence era. Chinese Journal of Biotechnology, 2022, 38(11): 4180-4199. (in Chinese) |
| [154] |
Heeres A S, Picone C S F, van der Wielen L A M, et al. Microbial advanced biofuels production: Overcoming emulsification challenges for large-scale operation. Trends in Biotechnology, 2014, 32(4): 221-229. DOI:10.1016/j.tibtech.2014.02.002 |